In a new study entitled “Autophagy is unregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase-1,” researchers describe significant activation of autophagy in ovarian endometriosis when compared to healthy controls, suggesting autophagy may underlie endometriosis pathogenesis. The study was published in the journal Fertility and Sterility.
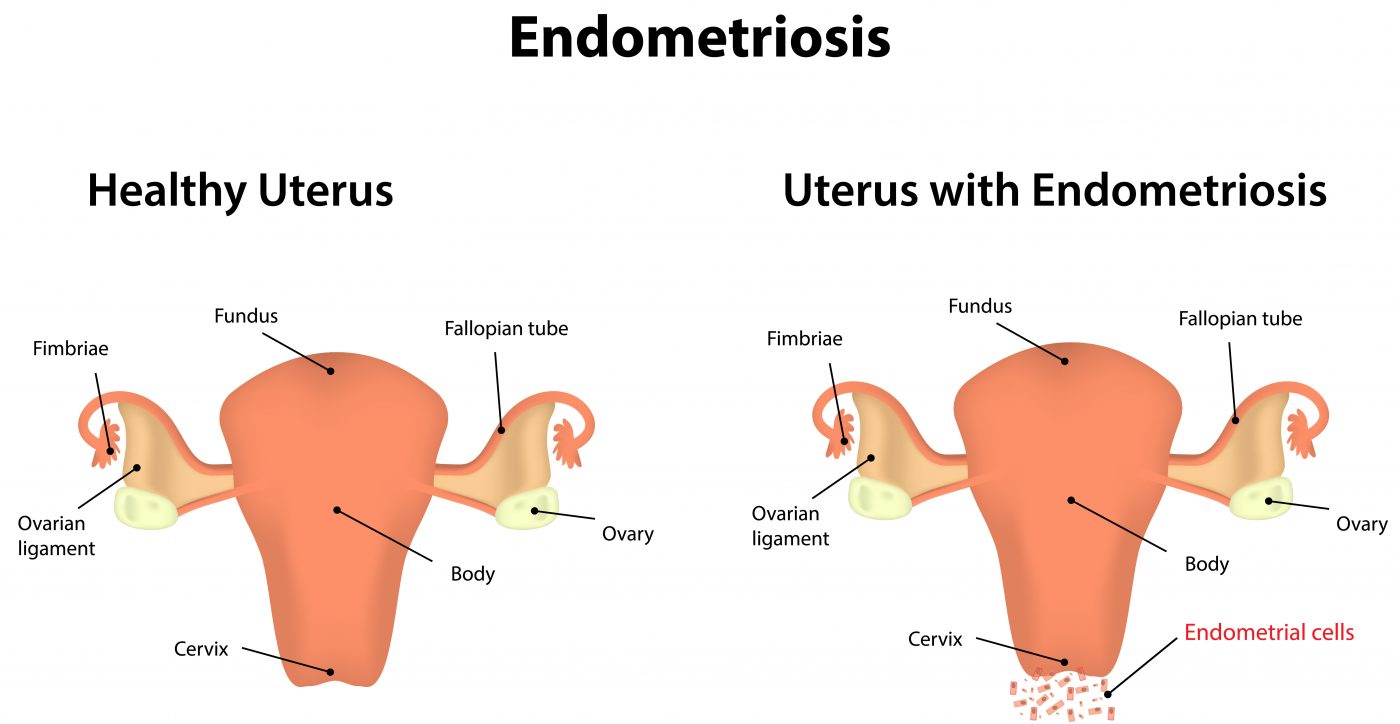
Endometriosis is a disease affecting 8 to 10% of fertile women and is characterized by cells of the inner lining of the uterus (the endometrium) colonizing the pelvic area, leading to proliferation outside of the uterus. Endometrial cells must escape apoptosis (programmed cell death) and in fact these cells were shown to increase their expression in antiapoptotic factors while exhibiting a decrease in the expression of proapoptotic factors.
In this study, the authors aimed to determine which mechanisms are responsible for the cells evading their normal context and proliferate by focusing on a process known as autophagy. Specifically in macro autophagy, cellular contents are degraded and then recycled. Notably, while an extended activation of autophagy can lead to cell death, in more moderate levels autophagy can actually promote cells’ survival upon stress conditions.
In this research, the authors determined how the authopagic process changes between women with normal endometrium when compared to women with ovarian endometriosis (when endometrium grows in ovaries). Additionally, in the endometriosis group the authors analyzed patients’ eutopic endometrium, i.e., the tissue in its correct place. To this end, the team analyzed samples of endometrium or endometriosis obtained surgically from patients with endometriosis and healthy controls. The activation of autophagy was measured by different techniques (including western blot analysis and quantitative real-time polymerase chain reaction) of different autophagy markers.
The authors found a significant activation of authophagy in the ovarian endometriomas samples when compared to patients own eutopic endometrium or healthy controls (denoted by an increased expression of all tested autophagy markers). In light of their results, authors suggest autophagy activation is necessary to not only allow cells to resist apoptosis as well as to promote their survival in ectopic places. This will contribute to lesion maintenance and thus they propose autophagy as a possible underlying mechanism driving endometriosis pathogenesis.