Inhibiting a certain enzyme improves endometriosis-associated symptoms and halts the growth of lesions in a mouse model of the disease, according to a study titled “Tranylcypromine, a lysine-specific demethylase 1 (LSD1) inhibitor, suppresses lesion growth and improves generalized hyperalgesia in mouse with induced endometriosis,” published in the open access journal Reproductive Biology and Endocrinology.
Endometriosis is a chronic inflammatory disease characterized by an abnormal growth of the endometrium (the tissue that normally lines the inside of a woman’s uterus) outside the uterine cavity. It affects roughly 6 to 10 percent of reproductive-age women, with symptoms ranging from infertility and dysmenorrhea to pelvic pain, significantly impairing patients’ quality of life. While initially described as a hormonal disease, endometriosis is also an inflammatory disorder with activation of inflammation genes and the release of pro-inflammatory cytokines.
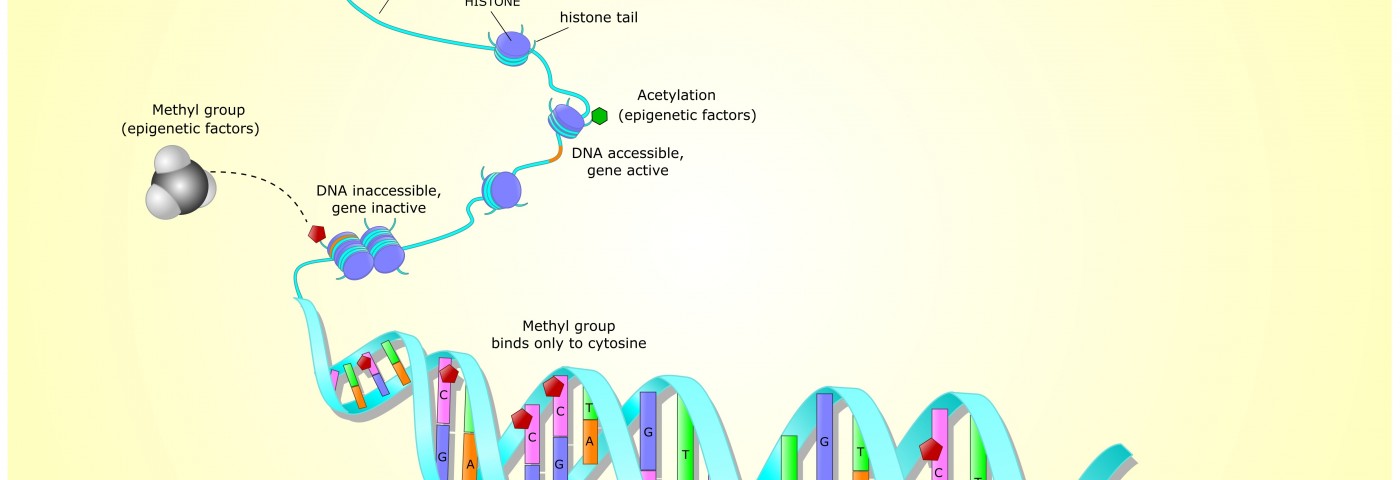
Endometriosis as an epigenetic disease is a concept that is increasingly gathering scientific evidence, and drugs designed to target the mechanism of epigenetic diseases (so-called “epi-drugs”) have proven their therapeutic potential to treat endometriosis, specifically histone deacetylases (HDACs) and DNA methyltransferases (DNMTs) in in vitro studies. However, studies to evaluate the potential of epi-drugs in preclinical settings are still lacking.
Previously, lysine-specific demethylase 1 (LSD1) expression, a key enzyme to alter epigenetic marks, was found aberrant in endometriosis, with in vitro studies revealing that treating endometriotic stromal cells with tranylcypromine, a LSD1 inhibitor, significantly reduced cellular proliferation, cell cycle progression, and invasiveness.
Researchers investigated the therapeutic potential of tranylcypromine in vivo. A total of 47 female mice were submitted to endometriosis-inducing surgery and then randomly divided into three groups – and untreated group, a low-dose TC group, and a high-dose TC group.
The team found that tranylcypromine treatment significantly reduced lesion size and improved generalized hyperalgesia (abnormally heightened sensitivity to pain) in a dose-dependent manner in mice with induced endometriosis. Moreover, tranylcypromine reduced immunoreactivity to biomarkers of proliferation and angiogenesis, impairing lesion growth.
These results, combined with the team’s previous findings of reduced cellular proliferation, cell cycle progression, and invasiveness upon LSD1 inhibition, support LSD1 as a promising new therapeutic target for endometriosis.