Researchers have demonstrated that platelets are a new player in endometriosis and are activated by endometriosis-derived stromal cells, contributing to disease development, according to a study titled “Endometriosis-Derived Stromal Cells Secrete Thrombin and Thromboxane A2, Inducing Platelet Activation” and published in the Reproductive Sciences journal. The findings may prompt the development of novel non-hormonal therapies for endometriosis.
Endometriosis is categorized mainly as an estrogen-dependent disease, leading to an estrogen-dependent growth of endometriotic lesions. Notably, endometriosis is also characterized by pelvic inflammation, with local release of proinflammatory cytokines and the recruitment and infiltration of immune cells, including macrophages and lymphocytes.
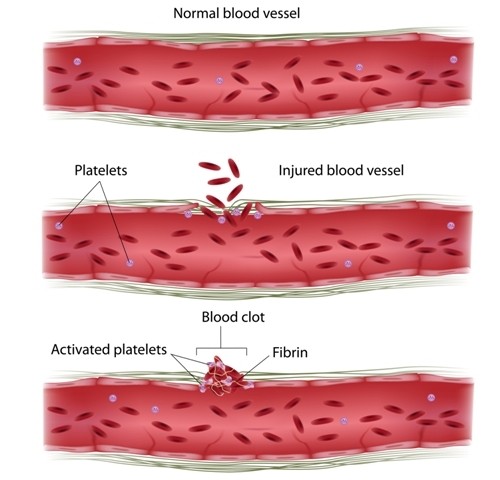
Platelets, also known as thrombocytes, are key blood components that work to stop bleeding by clumping and clotting blood vessel injuries. Emerging evidence indicates that coagulation and inflammation regulate each other, and platelets are increasingly recognized as inflammatory effector cells.
Following previous research in which researchers found that platelets aggregate in endometriotic lesions, expressing high levels of vascular endothelial growth factor (VEGF), the question tackled in the present study was what causes platelet aggregation in endometriotic lesions. Particular focus was placed on whether endometriotic lesions themselves secrete any inducers of platelet activation. To this end, researchers used primary ectopic endometrial stromal cells (EESCs) and platelets from healthy male volunteers to determine the length of platelet aggregation and activation.
The researchers found that endometriosis-derived stromal cells can induce platelet activation and aggregation. Later, the team determined the concentration of thromboxane (TX) B2 (TXB2), a metabolite of thromboxane A2 (TXA2), and the activity of thrombin, both potent inducers of platelet aggregation, and the two produced by endometriosis-derived stromal cells. They found that endometriosis-derived stromal cells secrete thrombin and TXA2, and induce platelet activation. Inhibiting thrombin with hirudin, a specific natural thrombin inhibitor, and using Ozagrel, a synthetic TXA2 inhibitor, resulted in significant suppression of platelet aggregation.
In conclusion, these results suggest that endometriosis-derived stromal cells induce platelet activation and aggregation by secreting coagulants, thrombin and TXA2. As a result, endometriotic lesions and platelets cross talk to promote the development of endometriosis. These observations further suggest that therapies based on non-hormonal, anticoagulations are a potential treatment for endometriosis, and may trigger the development of novel biomarkers for the disease.