Vermillion, Inc., an Austin, Texas-based bio-analytical solutions company, announced the appointment of a seven-member Steering Committee for its new Pelvic Mass Registry to advance the early detection and treatment of gynecologic diseases.
Vermillion, with a corporate focus on the discovery, development, and commercialization of high-value diagnostic and bio-analytical solutions for patients with gynecologic diseases, notes that pelvic masses can present a challenge to physicians and other healthcare professionals working to diagnosis and manage such diseases. Once pregnancy has been ruled out, for example, other forms of pelvic masses can include uterine, fallopian tube, or ovarian cancers. Masses can also invade the gastrointestinal and urinary tracts, such as in endometriosis.
The Pelvic Mass Registry’s target enrollment is 3,000 women, and will help to enable unprecedented multivariate diagnostic and “big data” algorithm research into the early detection, diagnosis, and treatment of various gynecologic conditions. Through monitoring women with suspicious or symptomatic pelvic masses, Vermillion anticipates being able to identify and measure diagnostic analytes, including DNA, RNA, and proteins, in addition to external diagnostic tools like imaging (like ultrasound, CT scan, or MRI) and clinical factors for the early detection, diagnosis, prognosis, and monitoring of the gynecologic diseases that annually afflict affect more than one million women in the United States.
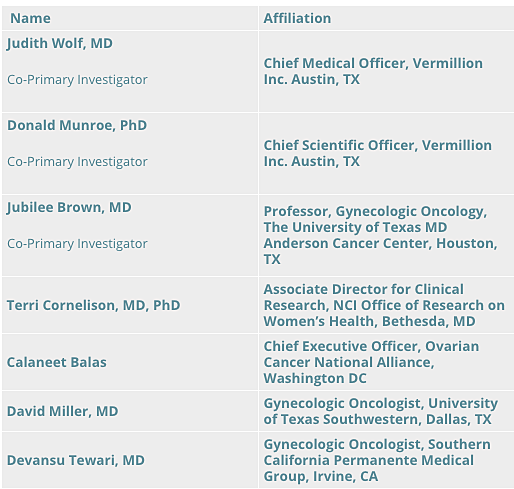
The Registry Steering Committee will consider and recommend scientific and clinical strategy to Vermillion, and oversee the design and publication of clinical studies. Committee members include national and international experts in the fields of ovarian cancer, endometriosis, and public health policy:
The Pelvic Mass Registry is the product of a public-private partnership, and Vermillion is to be awarded a $7.5 million grant from the Cancer Prevention and Research Institute of Texas (CPRIT) to support the registry. Vermillion’s receipt of the grant award is subject to mutually satisfactory execution and completion of a Vermillion and CPRIT contract, and may include such provisions as payment by Vermillion to CPRIT of future product royalties.
Since its establishment in 2009, CPRIT has awarded $1.24 billion in grants to Texas researchers, institutions, and organizations through its academic research, prevention and product development research programs. Programs made possible with CPRIT funding have reached all of the state’s 254 counties, brought more than 80 distinguished researchers to Texas, advanced scientific and clinical knowledge, and provided nearly $2 million in education, training, prevention and early detection services to Texans. For more information about CPRIT, visit: http://cprit.state.tx.us
 “Today’s Registry Steering Committee kickoff completes the first step of an ambitious plan to improve early detection and differential diagnosis of pelvic mass diseases requiring surgery,” says Vermillion’s chief medical officer and Registry co-primary investigator, Dr. Judith Wolf, in a release. “By sifting patient data and clinical samples for innovative diagnostic patterns, we hope to re-engineer the methods physicians use to sort and treat these conditions. Improved detection, diagnostic accuracy and timely access to specialist care benefits patients, physicians and healthcare systems alike.”
“Today’s Registry Steering Committee kickoff completes the first step of an ambitious plan to improve early detection and differential diagnosis of pelvic mass diseases requiring surgery,” says Vermillion’s chief medical officer and Registry co-primary investigator, Dr. Judith Wolf, in a release. “By sifting patient data and clinical samples for innovative diagnostic patterns, we hope to re-engineer the methods physicians use to sort and treat these conditions. Improved detection, diagnostic accuracy and timely access to specialist care benefits patients, physicians and healthcare systems alike.”
 “Vermillion’s Pelvic Mass Registry represents a major strategic initiative for the development of ‘big data’ algorithms to fight the war against ovarian cancer,” says Vermillion’s president and chief executive officer Valerie Palmieri. “In addition to malignancies,” Palmieri notes, “several other major debilitating benign conditions, such as endometriosis, which is equally difficult to diagnose and affects more than 14 million women per year and costs healthcare systems billions each year, will be incorporated into this one of a kind study. We are grateful for the commitment and support of the dedicated physicians, gynecologic oncology leaders and women’s health advocates of our Registry Steering Committee.”
“Vermillion’s Pelvic Mass Registry represents a major strategic initiative for the development of ‘big data’ algorithms to fight the war against ovarian cancer,” says Vermillion’s president and chief executive officer Valerie Palmieri. “In addition to malignancies,” Palmieri notes, “several other major debilitating benign conditions, such as endometriosis, which is equally difficult to diagnose and affects more than 14 million women per year and costs healthcare systems billions each year, will be incorporated into this one of a kind study. We are grateful for the commitment and support of the dedicated physicians, gynecologic oncology leaders and women’s health advocates of our Registry Steering Committee.”
Dr. Wolf has co-authored 94 peer-reviewed research articles, been principal investigator or co-investigator for 11 research grants related to gynecologic cancers, served as principal investigator or collaborator on 84 protocols, and has presented at more than 50 conferences, as well as at numerous scientific exhibitions and seminars. Dr. Wolf has been an adjunct professor of gynecologic oncology at The University of Texas MD Anderson Cancer Center and a clinical professor of the Division of Clinical Education at Arizona College of Osteopathic Medicine, as well as chairman of the Medical and Scientific Advisory Board of the National Ovarian Cancer Coalition. In addition, she is a member of the American Association of Cancer Research, Felix Rutledge Society, Society of Gynecologic Oncology, American Society of Clinical Oncology, and the American Gynecological and Obstetrical Society, ans is a Fellow of the American College of Obstetrics and Gynecology.
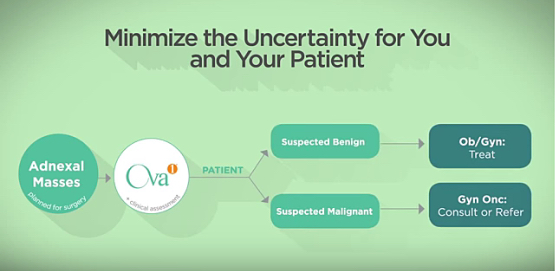
Vermillion’s lead diagnostic product in the United States is OVA1, a blood test for pre-surgically assessing ovarian tumors to determine whether they are malignant.
OVA1 is based on an innovative algorithmic approach and marketed through Vermillion’s wholly owned subsidiary, ASPiRA Labs. Vermillion says OVA1, which was the first FDA-cleared, protein-based in-vitro diagnostic multivariate index assay, represents a new class of software-enabled liquid biopsy diagnostics that minimize the uncertainty of the pre-surgical adnexal mass work-up.
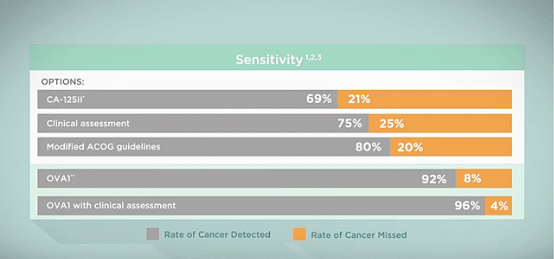
Vermillion says that by use of five biomarkers and the individual patient’s menopausal status, the OVA1 test can help detect more ovarian cancer than standard testing protocols, potentially initiating surgery by a cancer specialist earlier in the disease’s progression.
OVA1 has been FDA cleared for women who meet the following criteria:
• Are over 18 years of age
• Have an ovarian mass
• Have surgery planned
• Have not yet been referred to a gynecologic oncologist
• Have not had cancer in the past five years; and
• Have a rheumatoid factor concentration
For additional information about OVA1, including published clinical trials, visit: http://www.vermillion.com, and http://vermillion.com/providers/ova-1/ova1/.
Note: 1. Myers ER, Bastian LA, Havrilesky LJ, et al. Agency for Healthcare Research and Quality (US); 2006 Feb. (Evidence Reports/Technology Assessments, No. 130.)
Sources:
Vermillion, Inc.
Cancer Prevention and Research Institute of Texas (CPRIT)