A distinct difference exists in the gene expression of the immune system’s inflammatory mediators, known as cytokines, within endometrial tissues in people with endometriosis, a finding that helps to explain how the disease spreads and produces inflammatory responses in this patient group.
The study, “Immune-inflammation gene signatures in endometriosis patients,” was published online in the journal Fertility and Sterility,
Inflammation in Endometriosis
The link between inflammation and endometriosis (EM) severity and progression is well-established. The American College of Obstetrics and Gynecologists considers inflammation a potential cause of damage to the eggs and fallopian tubes leading to infertility in this patient population.
Although the research literature on this inflammatory link is rich, there is a dearth of understanding in regards to the precise molecular profiles of the genes that are involved in this process.
About this Study
Researchers looked at expression of 579 genes involved in immunology and inflammation in pelvic tissues — ectopic (outside of), eutopic (within), and endometrial — from eight infertile endometriosis patients with stage III-IV disease, compared with similar tissues from eight fertile and disease-free women undergoing tubal ligation, who served as controls.
Each tissue type was matched by menstrual cycle, with a particular focus on cytokine expression and its link to apoptosis (cell death) and EM lesion severity.
The process of gene expression is how the information that is stored within your genes is used and turned into a function. An example would be having genes for blue eyes and then being born with blue eyes because those genes were expressed during development.
Results revealed that eutopic endometrium of EM patients displayed a distinct molecular profile when compared with the control endometrium, specifically in genes involved in the regulation of apoptosis and decidualization (changes in the uterus due to progesterone during menstration). A total of 396 out of 579 screened immune and inflammation genes were significantly different in patient ectopic tissues compared with control endometrium.
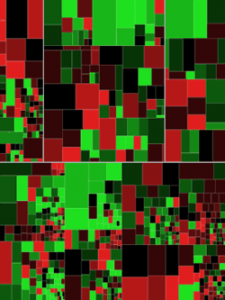
Heat mapping, a data visualization technique in which an array of colors are used to depict differential gene expression, revealed that the ectopic tissue samples were distinctly different from the matched eutopic endometrium and control endometrium, even within the same patient, providing evidence that in this patient population, the immune inflammatory mediation process is distinct on a molecular level.
“To our knowledge, this study is the first to attempt molecular profiling of differential immune and inflammation gene expression between matched ectopic tissues and eutopic endometrium from patients, ectopic and control endometrium, and eutopic and control endometrium that are also matched by menstrual cycle,” the authors wrote.
“Our results demonstrate dysregulation of a number of specific pathways. [O]ur data also provide explanation, evident from molecular profiling, of why endometrial fragments of women with endometriosis are able to implant, proliferate, establish localized blood supply, and elicit inflammatory stimulus,” they added.
The authors acknowledged that the sample population in this study was low, so future confirmatory studies with a larger sample size is needed.