University of South Florida researchers have showed that in a mouse model of endometriosis, normal body processes fail to properly regulate both ectopic and eutopic endometrium. And, they found, the anti-malaria drug hydroxychloroquine (HCQ) has potential as a therapeutic agent for women afflicted with the disease.
The study, “Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis,” by A. Ruiz and colleagues, was published in the online edition of the journal Cell Death and Disease.
Autophagy is the process of degradation of unnecessary or dysfunctional cellular parts, keeping cells free from debris. A cellular vesicle – the autophagsome – engulfs components to be destroyed and delivers them to the lysosome, which breaks down the components and recycles the pieces.
The research team used both cultured cells obtained from patients with endometriosis and a mouse model of the disease to study the autophagocytic processes in healthy endometrial cells and in cells from endometriotic lesions. By using HCQ – a compound with the ability to block autophagic flux – the team could observe what happens when autophagy was disrupted.
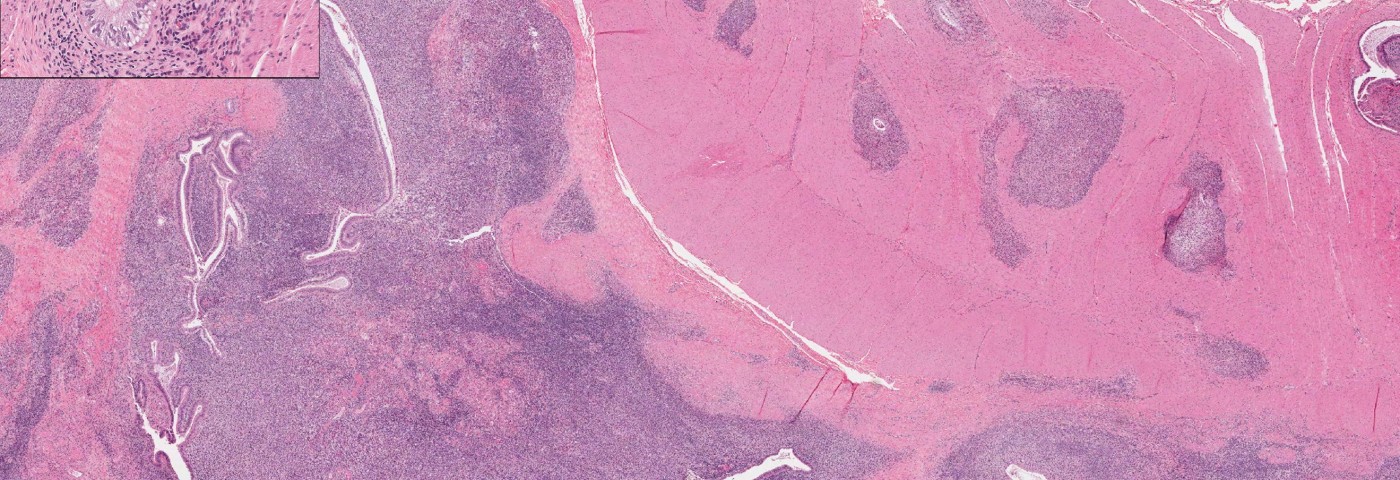
The mice were injected with fragments of the uterine horn, a process leading to endometriosis development within 14 days. The team observed that HCQ reduced the number of endometriotic lesions and altered the cellular organization within these tissues.
They also noticed that the inflammatory response in the endometriotic mice was altered by HCQ, with increased numbers of macrophages in HCQ-treated mice compared with control mice.
The researchers then went on to characterize the tissue organization following HCQ treatment, and observed that HCQ alters the organization of both normal endometrium and ectopic growths in the mouse model of endometriosis.
Since the protein expression of autophagic mediators was dysregulated in the endometriotic lesions, the data suggests that autophagy is dysregulated in the eutopic endometria of endometriosis-induced mice. Interestingly, autophagy was not affected by treatment with HCQ in the majority of tissues analyzed, including uterine horns.
“Additional studies will be required to determine how autophagy is regulated to allow development of endometriosis. Our results also show potential for a novel therapeutic approach for treating patients with this disease,” the author conclude.