The Woodlands, Texas, based firm Repros Therapeutics Inc. a development stage biopharmaceutical company focused on bringing to market new drugs focused on development of small molecule drugs for major unmet medical needs for treating male and female hormonal and reproductive system disorders, reports that the FDA has accepted an Investigational New Drug Application for its pipeline drug candidate vaginally delivered Proellex.
Repros describes Proellex as a new proprietary chemical entity that acts as a selective hormonal and reproductive system disorders receptor blocker that is being developed for treatment of symptoms associated with uterine fibroids and endometriosis. Approximately 20 percent of women of reproductive age in the U.S. have symptomatic fibroids, with roughly 300,000 hysterectomies performed annually as a consequence, and the National Uterine Fibroids Foundation estimates that 80 percent of all women in the U.S. have uterine fibroids by age 50, with one in four experiencing symptoms severe enough to require treatment. There are currently no FDA-approved chronic drug treatments for the long-term treatment of either of these conditions.Endometriosis is an often-painful disorder of unknown cause that occurs when cells of the tissue that normally forms the lining of the uterus — the endometrium — migrates to grow in in a location outside of the uterus — most commonly in other organs of the pelvis: ovaries, vagina, cervix, bladder, bowels, rectum or the tissue lining the pelvis. Rarely, endometriosis implants can manifest outside the pelvis, such as on the liver, in old surgery scars, and in extremely rare instances in or around the lungs or brain. These “colonies,” which are not cancerous, are called endometriosis implants, and can cause pain, heavy bleeding, bleeding between periods. They are also associated with infertility, although while he disorder is more common in women who are infertile than in fertile women, endometriosis is believed a to not necessarily cause infertility.
Every month, a woman’s ovaries produce hormones that tell the cells lining the uterus to swell and get thicker until the uterus sheds these cells along with blood and tissue through your vagina when the woman has her monthly menstrual period. Like uterine cells, endometriosis growths react to ovarian hormones growing and bleeding during menstrual periods, and over time, implants may add more tissue and blood, the buildup of which leads to pain and other symptoms. When endometriosis occurs in the ovaries, cysts called endometriomas may develop, and surrounding tissue may become irritated, eventually developing scar tissue and adhesions.
 While as noted above, the cause of endometriosis is unknown, some researchers think it is an autoimmune disorder. It also tends to run in families, so an element of heritability may be in play. Risk factors for endometriosis include having a mother or sister with endometriosis, early onset of menses, never having had children, frequent periods or menstruation lasting seven or more days, having a closed hymen, which blocks the flow of menstrual blood during the period.
While as noted above, the cause of endometriosis is unknown, some researchers think it is an autoimmune disorder. It also tends to run in families, so an element of heritability may be in play. Risk factors for endometriosis include having a mother or sister with endometriosis, early onset of menses, never having had children, frequent periods or menstruation lasting seven or more days, having a closed hymen, which blocks the flow of menstrual blood during the period.
The current standards of care for uterine fibroids and endometriosis consist of surgery or short-term treatment with gonadotropin-releasing hormone (“GnRH”) agonist drugs. GnRH agonists induce a low estrogen, menopausal-like state and promote bone loss and are not recommended for use for more than six months.
Repros’ Proellex-V is a proprietary vaginal delivery formulation of telapristone acetate, the drug’s active ingredient. Telapristone acetate is an anti-progestin that opposes the action of the female hormone progesterone. When an effective oral dose of telapristone is administered to a woman, she stops menstruating, with this cessation of menses having an obvious direct effect on symptoms such as excessive menstrual bleeding associated with uterine fibroids, and painful menses associated with endometriosis. At the same time, unlike drugs that suppress estrogen production, a woman’s ovaries continue to produce levels of estrogen that maintain bone mineral density while she is receiving telapristone. Telapristone acetate has been shown to significantly suppress the proliferative effect of progesterone on progesterone sensitive tissues as well as increase pro-apoptotic events making it an ideal drug candidate to shrink progesterone sensitive tumors such as uterine fibroids.
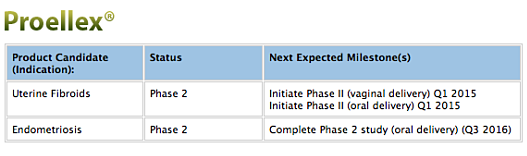
A Phase 2 Interventional, Randomized Double Blinded clinical Safety/Efficacy Study to Evaluate the Safety and Efficacy Proellex Administered Vaginally in the Treatment of Uterine Fibroids (ClinicalTrials.gov Identifier: NCT02323646) is currently underway at nine locations across the U.S. The primary objective of this study — Official Title: “A Phase 2, Multi-Center, Parallel Design, Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of 6 and 12 mg Proellex (Telapristone Acetate) Administered Vaginally in the Treatment of Premenopausal Women With Confirmed Symptomatic Uterine Fibroidsis” — is to determine the safety and efficacy of two vaginal doses of Proellex administered for up to 2 courses of treatment (18 weeks each), each separated by an Off-Drug Interval (ODI), to premenopausal women with symptomatic uterine fibroids. The primary Outcome Measures are to determine the percentage of subjects who become amenorrheic after one course of treatment. Estimated Primary Completion Date for the study is December 2015 (Final data collection date for primary outcome measure)
In a 2013 Phase 2 study of vaginally administered Proellex in the treatment of symptomatic fibroids, results suggested a 12 mg dose may provide clinical benefit to women suffering from symptomatic uterine fibroids. The primary endpoint in that study was change in Pictorial Blood Loss Assessment (PBAC). Secondary endpoints included change in Uterine Fibroid Symptom Quality of Life (UFSQOL) and change in fibroid volume by MRI assessment.
A total of 40 women were enrolled in the 2013 study into one of four groups, 3 mg (n=9), 6 mg (n=9), 12 mg (n=12) and 24 mg (n=10). The following table reports the data from those subjects that completed the study. Unexpectedly, the 24 mg dose exhibited lower exposure than the 12 mg dose which, Repros believes may result from the hydrophobic nature of the active ingredient and the low volume of excipient used in order to prevent vaginal leakage. Based on these findings, the Company decided to only pursue the 12 mg dose in Phase 3.
Reduction in fibroid volume was significant for the 12 mg dose as compared to either the 3 or 6 mg dose group (p=0.002 and p=0.01, respectively), with Repros observing that the magnitude of change and statistical significance were remarkable given the small number of subjects enrolled in the study. Among the women on the 12 mg. dose, 58 percent ceased menstruation, whereas 18 percent of the combined 3 and 6 mg doses stopped. Repros said it believed the incomplete cessation of menses at the 12 mg dose was a result of sporadic (poor compliance) administration of the drug in some of the subjects which was confirmed by evidence, on occasion, of no trough levels of active drug in those individuals that did not cease menstruation.
A majority of the subjects completed the study, and there were no discontinuations due to adverse events, although one serious adverse event was reported for one subject in the 6 mg dose group. Based on trough level analysis of active drug at every clinical visit, this subject sporadically administered the drug to herself, and under this partial progesterone antagonism Repros believed the uterine bleeding associated with the fibroids was exacerbated. This increased bleeding led to an anemic condition which resulted in the subject requiring blood transfusion. The subject was placed on a progestin and the condition resolved without further intervention. This finding suggested need for daily administration of the drug and also supports the 12 mg dose as the lowest dose for further development. Even though some women on the 12 mg dose exhibited no detectable trough levels at some of the clinical visits, the higher maximum concentrations of the drug when administered seemed to offset the risk of partial progesterone antagonism.
Repros Therapeutics Inc. holds exclusive worldwide rights to a composition of matter patent covering telapristone acetate. The Company also holds other issued and pending patents associated with its Proellex family of technologies. For more information about, Repros Therapeutics Inc. and Proellex visit:
http://www.reprosrx.com/proellex.php
In January of this year, Global Markets Direct published its Uterine Leiomyoma (Uterine Fibroids) Pipeline Review, H1 2015′, providing an overview of the Uterine Leiomyoma (Uterine Fibroids)’s therapeutic pipeline. This report provides comprehensive information on the therapeutic development for Uterine Leiomyoma (Uterine Fibroids), complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type, along with latest updates, and featured news and press releases. It also reviews key players involved in the therapeutic development for Uterine Leiomyoma (Uterine Fibroids) and special features on late-stage and discontinued projects. The report can be accessed at:
http://bit.ly/1INvCPl
Sources:
Repos Therapeutics Inc.
clinicalTrials.gov.
Global Markets Direct
Research and Markets
MedLine Plus
Mayo Clinic
MedicineNet

