Illinois-based AbbVie, in cooperation with Neurocrine Biosciences of San Diego, California, has announced positive top-line results from the second of two replicate Phase 3 clinical trials evaluating the efficacy and safety of their drug candidate Elagolix in premenopausal women who suffer pain from the gynecological disorder endometriosis.
Elagolix is a novel, orally administered formulation of gonadotropin-releasing hormone or GnRH antagonist, believed to exert its effect by altering the level of pituitary GnRH suppression and, as a result, titrating circulating estrogen levels. By this mechanism, it is believed that Elagolix will provide relief from pain associated with conditions such as endometriosis and uterine fibroids without need for active management of bone loss.
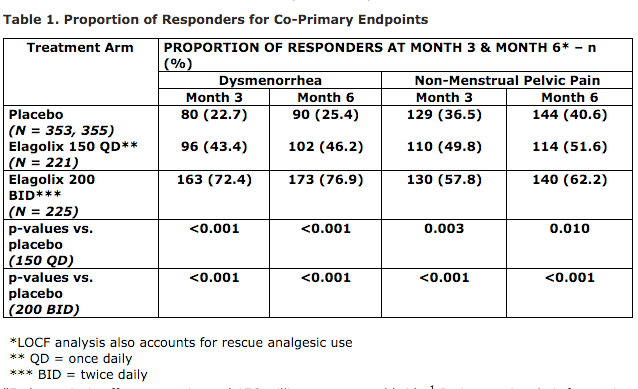
AbbVie reports preliminary results from the currently ongoing second Phase 3 trial showing that after six months of continuous treatment with Elagolix at two different doses (150 mg. once daily and 200 mg. twice daily). Both dosage levels met the study designers’ co-primary endpoints. Elagolix reduced menstrual pain (dysmenorrhea, DYS) scores as well as endometriosis associated non-menstrual pelvic pain (NMPP), at months three and six as measured by the Daily Assessment of Endometriosis Pain scale. The companies note that co-primary endpoint rates found in this second Phase 3 pivotal clinical trial are consistent with results from the previous Phase 3 study of Elagolix’s efficacy and safety.
“Endometriosis affects an estimated 176 million women worldwide. Patients voice their frustration about the need for more treatment options to medically manage endometriosis and its often debilitating pain,” said Michael Severino, MD (Photo Source Equilar), AbbVie executive vice president of Research and Development and chief scientific officer, in an AbbVie release, citing a World Endometriosis Research Foundation (WERF) metric.
“In an effort to address this need, AbbVie conducted the largest clinical trials in endometriosis to date. We are pleased with the outcomes of the pivotal trials thus far. AbbVie will continue to pursue Elagolix as a potential new treatment for the disease’s most common symptoms, including pain related to menstruation and chronic pelvic pain throughout the menstrual cycle.” (See Mayo Clinic Diseases and Symptoms: Endometriosis Fact Sheet for more information about endometriosis and pelvic pain.)
AbbVie, a global, research-based biopharmaceutical company spun off from Abbott Laboratories in 2013, and Neurocrine Biosciences — a biopharmaceutical company founded in 1992 specializing in therapies for neurological and endocrine diseases and disorders — also report that the Elagolix safety profile in this second Phase 3 trial is consistent with observations from the earlier Phase 3 pivotal study and with Elagolix studies prior to that.
The most common treatment-emergent adverse events (TEAEs) reported were hot flush, headache, and nausea. As anticipated by the mechanism of action, some adverse effects, such as hot flash, other hypoestrogenic TEAEs and changes in bone mineral density (BMD) were dose-dependent. Overall, they report that discontinuation rates were consistent across treatment groups (25.3 percent, 21.2 percent, and 19.7 percent for placebo, 150 mg. once daily and 200 mg. twice daily, respectively); while discontinuations specifically due to TEAEs were 6.1 percent, 4.4 percent, and 10.0 percent for placebo at dosages of 150 mg. once daily and 200 m.g twice daily, respectively.
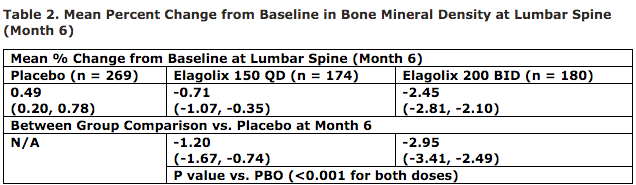
The mean percentage of change from baseline in BMD at the lumbar spine (LS) at month six is shown in Table 2 below, and AbbVie notes that the BMD finding for Elagolix 150 mg. once-daily is also similar to observations made in prior Phase 2 studies and an expected dose-dependent effect is noted for Elagolix 200 mg. twice-daily.
These preliminary results represent a six-month, group-level analysis. Participants in the Phase 3 trial will continue with either post-treatment follow-up or as part of a blinded six-month extension study to provide additional evaluation of Elagolix safety and efficacy. AbbVie says it also intends to present detailed results from the two Phase 3 trials at an unspecified 2016 medical conference. To date, Elagolix has been the subject of more than 40 clinical trials totaling more than 3,000 subjects, including several in patients with endometriosis and uterine fibroids, and AbbVie says it will now work on completing a clinical database in final preparation of a New Drug Application submission for Elagolix as a treatment for endometriosis anticipated in 2017.
Endometriosis
According to The World Endometriosis Research Foundation, the Mayo Clinic, and other authorities, endometriosis is a disorder in which tissue similar to the lining inside the uterus (called the endometrium), is found in colonies or “implants” outside the uterus, where it induces a chronic inflammatory reaction that may result in scar tissue. Endometriosis is primarily found on the pelvic peritoneum, on the ovaries, in the recto-vaginal septum, on the bladder, and in the bowel.
Endometriosis is associated with an array of symptoms, the most common being pain related both to menstruation as well as chronic pelvic pain throughout the menstrual cycle, and is a leading cause of infertility. WERF estimates that endometriosis affects an estimated one in 10 women during their reproductive years (usually between the ages of 15 and 49), which it calculates to approximately 176 million women worldwide suffering from the condition, with annual direct and indirect costs related to endometriosis estimated to exceed $20 billion in the United States alone.
There is currently no known cure for endometriosis, and while the disease can be treated and managed effectively with drugs such as oral contraceptives, NSAIDs, opioids, and GnRH agonists, most of these treatments are unsuitable for long-term use due to side effects. WERF notes that surgical interventions (such as laparotomy or laparoscopy) can be effective to remove endometriosis lesions and scar tissue, but success rates are dependent on the extent of disease and the individual surgeon’s skills, and often need to be performed repeatedly.
Clinical Trial Design
The first AbbVie Phase 3 trial (M12-665), a 24-week, randomized, double-blind, placebo-controlled study, evaluated Elagolix’s safety and efficacy in 872 women ages 18 to 49 who were experiencing moderate-to-severe endometriosis-associated pain. The trial was conducted at approximately 160 sites in the U.S., Puerto Rico, and Canada.
This second Phase 3 trial (M12-671) employed a design similar to that of the first Phase 3 pivotal trial, but was multinational in scope, involving a study of 815 women with moderate-to-severe endometriosis-associated pain across 226 sites in 13 countries (the U.S. and 12 others). These two Phase 3 pivotal studies evaluated the safety and effectiveness of Elagolix in nearly 1,700 women who had experienced endometriosis-associated pain, which AbbVie says represents the largest prospective randomized endometriosis treatment trials ever conducted.